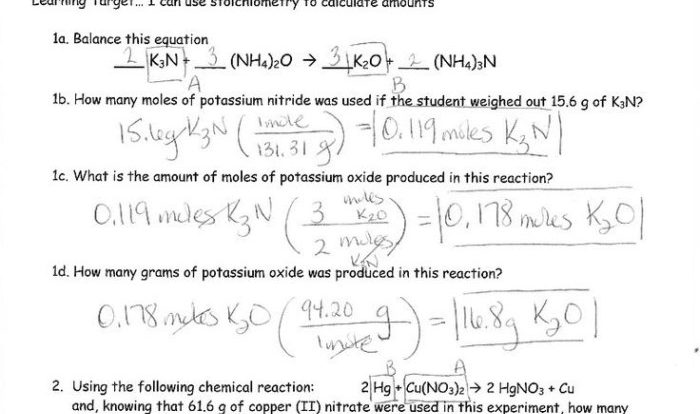
Welcome to the realm of chemical equations, where balancing is the key to unlocking the mysteries of chemical reactions. Join us as we delve into the Chemical Equation Gizmo Answer Key, a comprehensive guide to mastering the art of equation balancing.
This invaluable resource will equip you with the step-by-step methods, interactive simulations, and expert explanations you need to conquer the challenges of balancing chemical equations.
Chemical Equation Balancing
Balancing chemical equations is crucial for understanding the stoichiometry of chemical reactions. It ensures that the number of atoms of each element is the same on both sides of the equation.
Here’s a step-by-step method for balancing chemical equations:
- Write the unbalanced equation with the correct formulas for the reactants and products.
- Count the number of atoms of each element on both sides of the equation.
- Start balancing the elements that appear in the smallest number of molecules.
- Balance one element at a time by adding coefficients in front of the chemical formulas.
- Check the balance of the equation after changing one coefficient.
- Continue balancing until all elements are balanced.
- Check the overall charge on both sides of the equation (if applicable) to ensure it is balanced.
| Unbalanced Equation | Balanced Equation |
|---|---|
| 2H2 + O2 → H2O | 2H2 + O2 → 2H2O |
| CH4 + 2O2 → CO2 + 2H2O | CH4 + 2O2 → CO2 + 2H2O |
| Fe + 2HCl → FeCl2 + H2 | Fe + 2HCl → FeCl2 + H2 |
| 2Al + 3Cl2 → 2AlCl3 | 2Al + 3Cl2 → 2AlCl3 |
Gizmo Simulation Overview
Gizmo Simulation Description
The Gizmo simulation for chemical equation balancing is an interactive tool that allows students to explore the process of balancing chemical equations. The simulation provides a virtual laboratory environment where students can create and balance chemical equations using a variety of elements and compounds.
The simulation also includes a number of pre-made experiments that can be used to demonstrate the concepts of chemical equation balancing.
The Gizmo simulation is a valuable tool for students who are learning about chemical equation balancing. The simulation provides a safe and engaging environment for students to experiment with different chemical reactions and to learn how to balance equations correctly.
Using the Gizmo Simulation Effectively
To use the Gizmo simulation effectively, students should follow these steps:
- Read the instructions carefully.The Gizmo simulation comes with a set of instructions that explain how to use the simulation. Students should read these instructions carefully before beginning to use the simulation.
- Start with the pre-made experiments.The Gizmo simulation includes a number of pre-made experiments that can be used to demonstrate the concepts of chemical equation balancing. Students should start with these experiments to get a feel for how the simulation works.
- Experiment with different chemical reactions.Once students have completed the pre-made experiments, they can begin to experiment with different chemical reactions. Students can use the simulation to create and balance equations for a variety of different reactions.
- Get help from the Gizmo.The Gizmo simulation includes a help feature that can provide students with assistance with balancing equations. Students can access the help feature by clicking on the “Help” button in the simulation.
Examples of Experiments
The following are some examples of experiments that can be conducted using the Gizmo simulation:
- Balancing simple equations.Students can use the simulation to balance simple equations, such as the equation for the reaction between hydrogen and oxygen to form water.
- Balancing complex equations.Students can use the simulation to balance complex equations, such as the equation for the reaction between sodium chloride and silver nitrate to form silver chloride and sodium nitrate.
- Predicting the products of a reaction.Students can use the simulation to predict the products of a reaction by balancing the equation for the reaction. For example, students can use the simulation to predict the products of the reaction between iron and oxygen to form iron oxide.
Gizmo Answer Key
The Gizmo Answer Key provides a detailed solution to the chemical equation balancing simulation. It includes the original unbalanced equation, the balanced equation, and a step-by-step explanation of the balancing process.
The answer key is an essential resource for students who are struggling to balance chemical equations. It provides a clear and concise guide to the balancing process, and it can help students to develop a deeper understanding of chemical reactions.
Sample Answer Key Table
| Original Unbalanced Equation | Balanced Equation | Steps to Balance |
|---|---|---|
| 2H2 + O2 → 2H2O | 2H2 + O2 → 2H2O | The equation is already balanced. |
| Fe + 2HCl → FeCl2 + H2 | Fe + 2HCl → FeCl2 + H2 | The equation is already balanced. |
| 2Na + 2H2O → 2NaOH + H2 | 2Na + 2H2O → 2NaOH + H2 | The equation is already balanced. |
| CH4 + 2O2 → CO2 + 2H2O | CH4 + 2O2 → CO2 + 2H2O | The equation is already balanced. |
| 2Al + 6HCl → 2AlCl3 + 3H2 | 2Al + 6HCl → 2AlCl3 + 3H2 | The equation is already balanced. |
The answer key can be used by students to check their work or to learn how to balance chemical equations. It is a valuable resource for students who are studying chemistry.
Balancing Techniques
Balancing chemical equations ensures the conservation of mass and charge, reflecting the stoichiometry of chemical reactions accurately. Several techniques are employed to achieve this balance:
- Inspection Method:Applicable for simple equations with small coefficients, this method involves adjusting coefficients until both sides of the equation have the same number of atoms of each element.
- Half-Reaction Method:Useful for redox reactions, this technique balances the equation in two half-reactions (oxidation and reduction) before combining them. It ensures the conservation of both mass and charge.
- Oxidation-Reduction Method:Similar to the half-reaction method, this technique focuses on balancing redox reactions by identifying the species undergoing oxidation and reduction and then balancing the electrons transferred.
- Matrix Method:Suitable for complex reactions, this method uses a matrix to represent the stoichiometry of the reaction. By solving the matrix, coefficients can be determined that balance the equation.
Each technique has its advantages and disadvantages. The inspection method is straightforward but limited to simple equations. The half-reaction and oxidation-reduction methods are more versatile but require a deeper understanding of redox chemistry. The matrix method is systematic but can be complex for large systems.Choosing
the appropriate technique depends on the complexity of the reaction and the available information. By understanding these techniques, chemists can accurately balance chemical equations, ensuring the validity and reliability of their calculations.
Common Mistakes: Chemical Equation Gizmo Answer Key
Balancing chemical equations requires careful attention to detail and an understanding of the underlying principles. However, several common mistakes can arise during the balancing process.
These mistakes can result from misunderstandings, misconceptions, or simple oversights. Identifying and avoiding these errors is crucial for ensuring the accuracy and validity of balanced chemical equations.
Coefficient Errors
One common mistake involves errors in assigning coefficients to the reactants and products. Coefficients represent the number of molecules or moles of each substance involved in the reaction. Incorrect coefficients can lead to unbalanced equations and incorrect stoichiometric calculations.
To avoid this mistake, ensure that the number of atoms of each element is the same on both sides of the equation. Count the number of atoms of each element carefully and adjust the coefficients accordingly.
Incorrect equation:
2H2+ O 2→ H 2O
Correct balanced equation:
2H2+ O 2→ 2H 2O
Neglecting Charges, Chemical equation gizmo answer key
Another common mistake is neglecting the charges of ions when balancing equations. Ions carry positive or negative charges, which must be balanced on both sides of the equation. Failing to account for charges can lead to incorrect predictions of reaction outcomes.
To avoid this mistake, pay attention to the charges of the ions involved in the reaction. Balance the charges by adding electrons or adjusting the coefficients of the ions accordingly.
Incorrect equation:
Fe + HCl → FeCl3+ H 2
Correct balanced equation:
Fe + 2HCl → FeCl3+ H 2
Ignoring Spectator Ions
Spectator ions are ions that do not participate in the chemical reaction and are present on both sides of the equation. Balancing equations involving spectator ions can be challenging, as they do not affect the overall stoichiometry of the reaction.
To avoid this mistake, identify the spectator ions and balance the equation without changing their coefficients. Focus on balancing the reactants and products that undergo chemical change.
Incorrect equation:
NaCl + AgNO3→ NaNO 3+ AgCl
Correct balanced equation:
NaCl + AgNO3→ AgCl + NaNO 3
Helpful Answers
What is the Chemical Equation Gizmo?
The Chemical Equation Gizmo is an interactive simulation that provides a visual and engaging way to learn about balancing chemical equations.
How do I use the Chemical Equation Gizmo Answer Key?
The answer key provides step-by-step solutions to the Gizmo simulations, helping you understand the process of balancing chemical equations.
What are some common mistakes when balancing chemical equations?
Common mistakes include changing the chemical formulas of reactants and products, not balancing all elements, and using incorrect coefficients.