Delving into the realm of chemistry, the empirical and molecular formula worksheet PDF emerges as an invaluable tool for students and educators alike. This comprehensive resource provides a structured framework for understanding the fundamental concepts of empirical and molecular formulas, empowering learners with the knowledge and skills to navigate the intricacies of chemical composition.
The worksheet is meticulously designed to guide users through the stepwise determination of empirical and molecular formulas, unraveling the relationship between these formulas and their applications in various scientific disciplines. With its clear instructions, illustrative examples, and practice problems, this worksheet fosters a deep understanding of the subject matter, making it an indispensable resource for effective chemistry education.
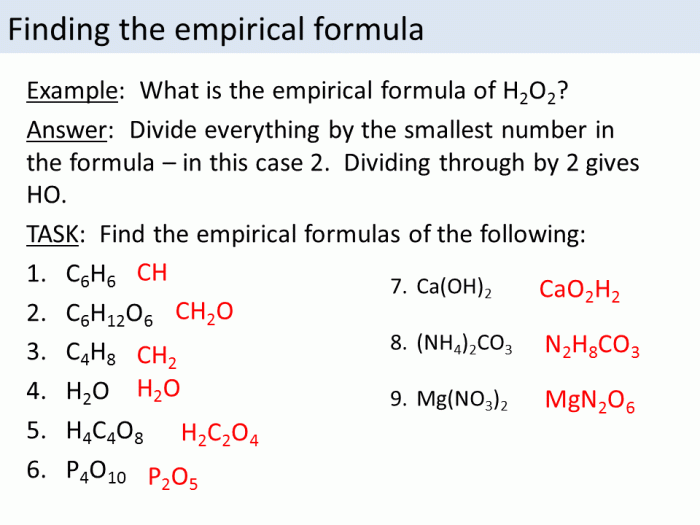
Empirical and Molecular Formulas: Empirical And Molecular Formula Worksheet Pdf

Empirical formulas and molecular formulas are essential in chemistry for understanding the composition and structure of compounds. They provide valuable information about the elements present and the relative proportions of atoms in a substance.
An empirical formula represents the simplest whole-number ratio of atoms or elements in a compound, while a molecular formula indicates the exact number of each type of atom in a molecule.
Determining Empirical Formula
To determine the empirical formula, the following steps can be followed:
- Convert the mass percentages of each element to grams.
- Convert the grams of each element to moles.
- Divide each mole value by the smallest number of moles to obtain the simplest whole-number ratio.
Determining Molecular Formula
The molecular formula can be determined by comparing the empirical formula to the molar mass of the compound. The following steps can be used:
- Multiply the empirical formula by an integer to obtain a formula with a molar mass close to the experimental molar mass.
- Check if the resulting formula is consistent with the empirical formula.
Applications of Empirical and Molecular Formulas, Empirical and molecular formula worksheet pdf
Empirical and molecular formulas have numerous applications in various fields, including:
- Chemistry: Identifying compounds, predicting properties, and understanding chemical reactions.
- Medicine: Developing drugs, understanding drug metabolism, and diagnosing diseases.
- Environmental science: Monitoring pollution levels, understanding environmental processes, and assessing environmental impact.
Expert Answers
What is the difference between an empirical formula and a molecular formula?
An empirical formula represents the simplest whole-number ratio of elements present in a compound, while a molecular formula specifies the exact number of atoms of each element in a molecule of the compound.
How can I determine the empirical formula of a compound?
The empirical formula can be determined by converting the mass percentages of elements in a compound to their corresponding mole ratios and then simplifying these ratios to the smallest whole numbers.
What is the relationship between the empirical formula and the molecular formula?
The molecular formula is a multiple of the empirical formula, and the multiplier is equal to the ratio of the molecular mass to the empirical formula mass.